Vorsetuzumab mafodotin
 | |
| Monoclonal antibody | |
|---|---|
| Type | ? |
| Source | Humanized (from mouse) |
| Target | CD70 |
| Clinical data | |
| Other names | SGN-75 |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
|
| ChemSpider |
|
| UNII |
|
| KEGG |
|
| Chemical and physical data | |
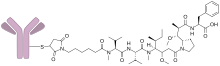
| Formula | C6476H10006N1726O2028S50(C49H78N6O11)3-5 |
| Molar mass | 150 kg/mol |
Vorsetuzumab mafodotin (SGN-75) is an antibody-drug conjugate (ADC) directed to the protein CD70 designed for the treatment of cancer.[1] It is a humanized monoclonal antibody, vorsetuzumab, conjugated with noncleavable monomethyl auristatin F (MMAF), a cytotoxic agent.[citation needed]
This drug was developed by Seattle Genetics, Inc. The drug completed phase I clinical trials for renal cell carcinoma,[2] but development was discontinued in 2013.[3] No reason was given but SG plan to start clinical trials of SGN-CD70A in 2014.[3]
References
- ^ Statement On A Nonproprietary Name Adopted By The USAN Council: Vorsetuzumab Mafodotin, American Medical Association.
- ^ Clinical trials for SGN-75 "Clinicaltrials.gov"
- ^ a b Seattle Genetics Third Quarter 2013 Financial Report[permanent dead link]