User:Mr. Ibrahem/Trospium
 | |
| Clinical data | |
|---|---|
| Trade names | Regurin, Sanctura, others[1] |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets, capsules) |
| Drug class | Antimuscarinic agent[2] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50–85% |
| Onset of action | Within 7 days[3] |
| Elimination half-life | 20 hours |
| Identifiers | |
| |
| Chemical and physical data | |
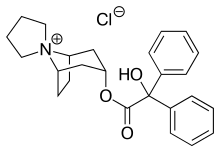
| Formula | C25H30ClNO3 |
| Molar mass | 427.97 g·mol−1 |
| 3D model (JSmol) |
|
| |
| |
| (verify) | |
Trospium, sold under the brand name Sanctura among others, is a medication used to treat symptoms of overactive bladder.[4] It is taken by mouth.[4] Effects begin within 7 days.[3]
Common side effects include dry mouth, abdominal pain, and constipation.[4][5] Other side effects may include urinary retention, agitation, and anaphylaxis.[4][5] Safety in pregnancy is unclear.[6] It is a antimuscarinic agent with minimal effects on the central nervous system.[2] It works by causing the smooth muscle in the bladder to relax.[7]
Trospium was patented in 1966 and approved for medical use in 1974.[8] It is available as a generic medication.[5] In the United Kingdom a month of medication costs the NHS about £6 in 2021.[5] In the United States this amount costs about 30 USD.[9]
References
- ^ Drugs.com international brands of trospium Archived 2019-10-22 at the Wayback Machine Page accessed May 13, 2016
- ^ a b Biastre K, Burnakis T (February 2009). "Trospium chloride treatment of overactive bladder". Ann Pharmacother. 43 (2): 283–95. doi:10.1345/aph.1L160. PMID 19193592. S2CID 20102756.
- ^ a b Kreder, Karl; Dmochowski, Roger (10 July 2007). The Overactive Bladder: Evaluation and Management. CRC Press. p. 193. ISBN 978-0-203-93162-2. Archived from the original on 23 September 2021. Retrieved 20 September 2021.
- ^ a b c d e f g "Trospium Monograph for Professionals". Drugs.com. Archived from the original on 20 January 2021. Retrieved 20 September 2021.
- ^ a b c d e BNF (80 ed.). BMJ Group and the Pharmaceutical Press. September 2020 – March 2021. p. 824. ISBN 978-0-85711-369-6.
{{cite book}}: CS1 maint: date format (link) - ^ "Trospium Use During Pregnancy". Drugs.com. Archived from the original on 3 December 2020. Retrieved 20 September 2021.
- ^ "Regurin XL 60mg - Summary of Product Characteristics (SmPC) - (emc)". www.medicines.org.uk. Archived from the original on 17 January 2021. Retrieved 20 September 2021.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 446. ISBN 9783527607495. Archived from the original on 2020-11-15. Retrieved 2021-03-16.
- ^ "Trospium Prices, Coupons & Patient Assistance Programs". Drugs.com. Archived from the original on 10 April 2021. Retrieved 20 September 2021.