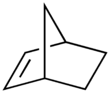
Norbornene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[2.2.1]hept-2-ene | |||
| Other names
Norbornylene
Norcamphene | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChEBI |
| ||
| ChemSpider |
| ||
| ECHA InfoCard | 100.007.152 | ||
| EC Number |
| ||
PubChem CID
|
| ||
| UNII |
| ||
CompTox Dashboard (EPA)
|
| ||
| |||
| |||
| Properties | |||
| C7H10 | |||
| Molar mass | 94.157 g·mol−1 | ||
| Appearance | White solid | ||
| Melting point | 42 to 46 °C (108 to 115 °F; 315 to 319 K) | ||
| Boiling point | 96 °C (205 °F; 369 K) | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | −15 °C (5 °F; 258 K) | ||
| Related compounds | |||
Related compounds
|
Nadic anhydride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity.
Production
Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene. Many substituted norbornenes can be prepared similarly.[2][3] Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene.
Reactions
Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbonion controversy.
Norbornene is used in the Catellani reaction and in norbornene-mediated meta-C−H activation.[4]
Certain substituted norbornenes undergo unusual substitution reactions owing to the generation of the 2-norbornyl cation.
Being a strained ene, norbornenes react readily with thiols in the thiol-ene reaction to form thioethers. This makes norbornene-functionalized monomers ideal for polymerization with thiol-based monomers to form thiol-ene networks.[5]
Polynorbornenes
Norbornenes are important monomers in ring-opening metathesis polymerizations (ROMP). Typically these conversions are effected with ill-defined catalysts. Polynorbornenes exhibit high glass transition temperatures and high optical clarity.[6]

ROMP reaction giving polynorbornene. Like most commercial alkene metathesis processes, this reaction does not employ a well-defined molecular catalyst.
In addition to ROMP, norbornene monomers also undergo vinyl-addition polymerization, and is a popular monomer for use in cyclic olefin copolymers.
Polynorbornene is used mainly in the rubber industry for antivibration (rail, building, industry), antiimpact (personal protective equipment, shoe parts, bumpers) and grip improvement (toy tires, racing tires, transmission systems, transports systems for copiers, feeders, etc.)
Ethylidene norbornene is a related monomer derived from cyclopentadiene and butadiene.
See also
References
- ^ Norbornene MSDS
- ^ Binger, Paul; Wedemann, Petra; Brinker, Udo H. "Cyclopropene: A New Simple Synthesis and its Diels-Alder Reaction with Cyclopentadiene". Organic Syntheses; Collected Volumes, vol. 10, p. 231.
- ^ Oda, Masaji; Kawase, Takeshi; Okada, Tomoaki; Enomoto, Tetsuya. "2-Cyclohexene-1,4-dione". Organic Syntheses; Collected Volumes, vol. 9, p. 186.
- ^ Thansandote, Praew; Chong, Eugene; Feldmann, Kai-Oliver; Lautens, Mark (21 May 2010). "Palladium-Catalyzed Domino C−C/C−N Coupling Using a Norbornene Template: Synthesis of Substituted Benzomorpholines, Phenoxazines, and Dihydrodibenzoxazepines". The Journal of Organic Chemistry. 75 (10): 3495–3498. doi:10.1021/jo100408p. PMID 20423091.
- ^ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1540–1573. doi:10.1002/anie.200903924.
- ^ Delaude, Lionel; Noels, Alfred F. (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01. ISBN 978-0471238966.