Methylenedioxyallylamphetamine
This article relies largely or entirely on a single source. (October 2017) |
| |
| Names | |
|---|---|
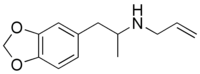
| Preferred IUPAC name
N-[1-(2H-1,3-Benzodioxol-5-yl)propan-2-yl]prop-2-en-1-amine | |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C13H17NO2 | |
| Molar mass | 219.284 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Methylenedioxyallylamphetamine (MDAL or 3,4-methylenedioxy-N-allylamphetamine) is a lesser-known psychedelic drug. It is also the N-allyl derivative of 3,4-methylenedioxyamphetamine (MDA). MDAL was first synthesized by Alexander Shulgin. In his book PiHKAL, the minimum dosage is listed as 180 mg, and the duration unknown.[1] MDAL produces few to no effects on its own, but may enhance the effects of LSD. Very little data exists about the pharmacological properties, metabolism, and toxicity of MDAL.
Legality
United Kingdom
This substance is a Class A drug in the Drugs controlled by the UK Misuse of Drugs Act.[2]
See also
References
- ^ MDAL entry in PiHKAL
- ^ "UK Misuse of Drugs act 2001 Amendment summary". Isomer Design. Archived from the original on 22 October 2017. Retrieved 12 March 2014.